
What if every PCR result came with a confidence meter?
For labs working in GMP, or CLIA settings, precision isn’t optional — it’s required. Reporting flawed data isn’t just a technical error — it’s a regulatory risk; a patient impacted; a failed QC batch. Yet even today, many PCR systems spit out raw counts with no measure of whether those results are trustworthy.
Enter the ID score—a new metric found only in Countable PCR that’s designed not to tell you what your PCR result is, but how much to trust it.
What is an ID score?
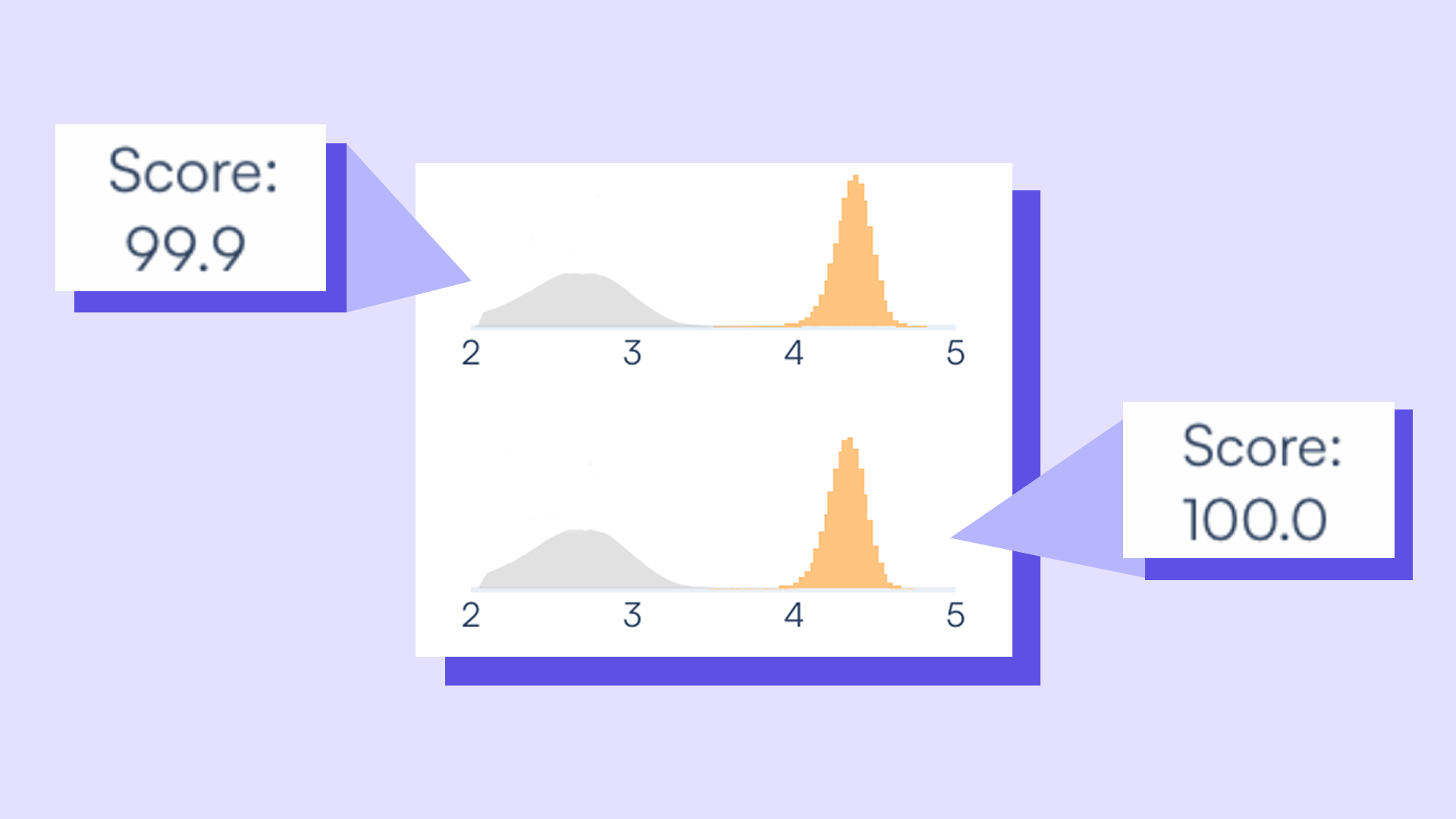
The Intensity Distribution (ID) score is a unit-less metric ranging from 0–100 that describes how clearly the fluorescence signal stands apart from the background in each sample. Think of it as a sharpness filter for your data — the higher the ID score, the better the separation between signal and noise.
That clarity matters. In Countable PCR, the ID score is automated, and it acts as a built-in quality check, quietly scanning for artifacts, sloppy separation, or noisy tails that muddy the analysis.
.png)
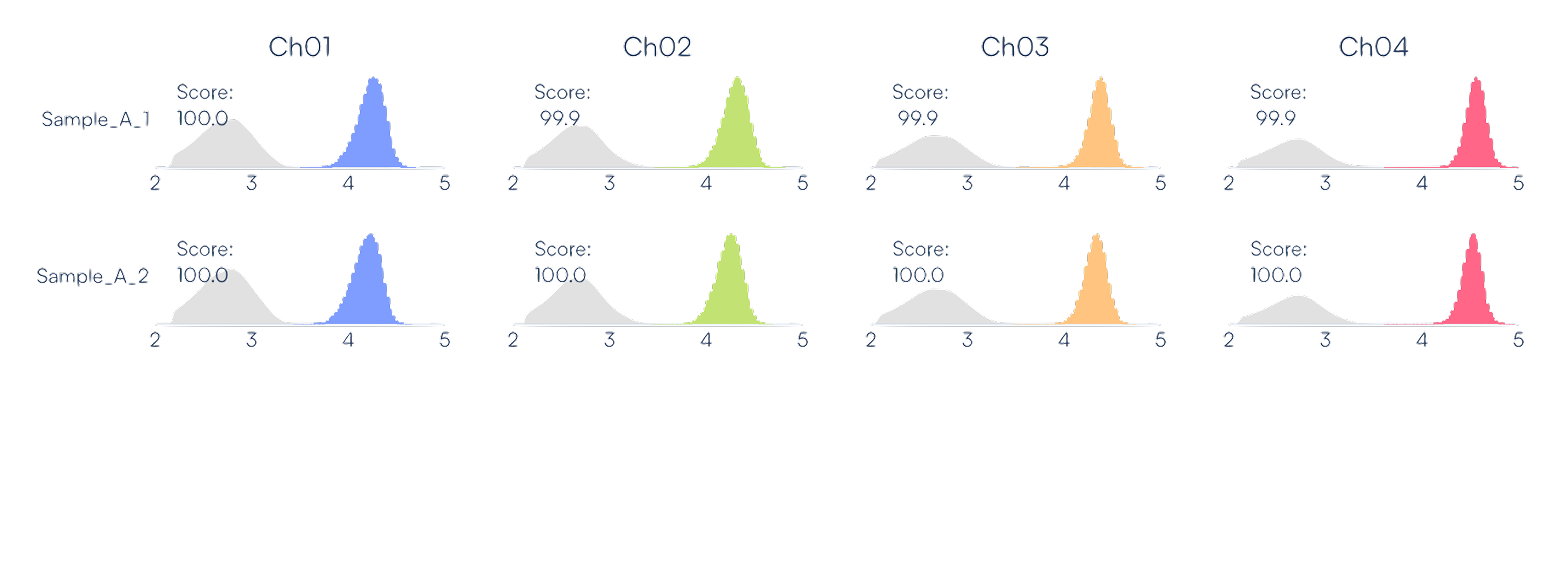
Real data output from Countable PCR. [top] For each sample, Countable PCR reports the number of targets counted in each channel as counts per 50 µL. [bottom] Each sample has a respective ID score that reports on the quality of the counts.
Why it matters in regulated environments
Auditing results in GMP and CLIA environments is required to ensure a good standard of care and reproducible experiments. That’s why automated, objective metrics like the ID score are a game-changer.
- For CGT labs, the ID score helps ensure batch-to-batch consistency, even when upstream variability creeps in.
- For QC professionals, it flags assay drift or PCR inhibition—without waiting for an out-of-spec count.
- For labs reporting to patients, it adds a layer of statistical confidence that supports clinical decisions and traceable documentation.
The ID score adds a layer of trust in your data that other platforms don’t offer. It’s a simple thing that supports your biggest responsibilities: patient outcomes, product quality, and regulatory trust.


.jpg)
.png)
