
Abstract
Proximal tubule (PT) cells are the most abundant cell type in the kidney cortex and critical for the re-absorption of water, ions, and small molecules like glucose and amino acids.These cells have a high demand for energy, and as a result have hundreds of mitochondria per cell. Mitochondrial copy number (mtCN) and DNA damage have been implicated as a source of dysfunction and decreased efficiency or even apoptosis in many cell types, including heart and brain cells. Here,we investigate the relationship between mitochondrial DNA (mtDNA)and nuclear DNA content as a method of assessing the role of mtCN in kidney function and aging.
mtDNA is present at up to hundreds of times greater levels compared to diploid nuclear DNA, which presents ac hallenge when trying to measure both targets in a single assay. Further, many current PCR-based assays do not allow for a level of sensitivity to make definitive conclusions about changes in mtCN.
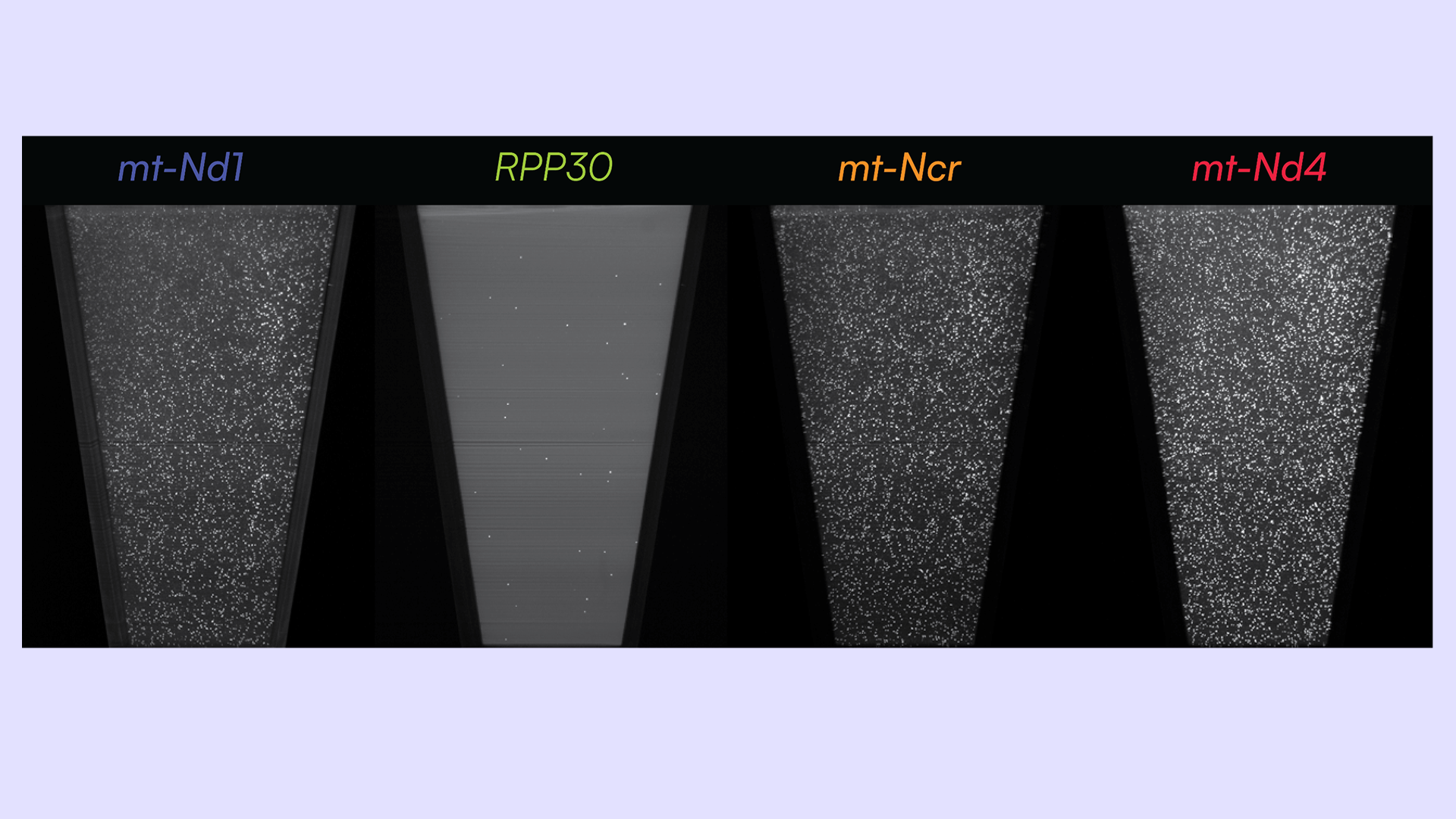
Here, we aim to develop a fast and conclusive PCR-based assay to compare the abundance of mtDNA alongside nuclear DNA in rat kidney cortex. We selected two mtDNA deletion hotspots implicated in aging and disease, mt-Nd4and mt-Ndr. These hotspots are associated with the emergence of heteroplasmy and decreased mitochondrial function. Additionally, we selected a more stable mtDNA target, mt-Nd1 and a nuclear genomic target, Rpp30, to use as reference regions.
To overcome challenges with comparing these targets, we designed a 4-plex assay to target mt-Nd1, mt-Ndr, mt-Nd4 and Rpp30 with Countable PCR. We were able to achieve:
- Accurate,direct counts of mitochondrial copies per cell, with comparison between highabundance mtDNA targets and low abundance targetnuclear genome targets in the same reaction
- Lower variability in individual results, revealing subtle biological shifts not previously measured, including the initial increase in mtDNA counts associated with adolescence followed by a decline with age
- 4-target multiplex results without extensive optimization – three days to draw definitive conclusions about mtCN
Together, these results are an important proof-of-concept for tracing key mtDNA targets implicated in kidney disease or deteriorating function from aging. With the ability to compare mtDNA targets directly against their nuclear gene counterparts, we open up new avenues for interrogating the relationship between mtCN and mtDNA damage in a variety of cell types.
Abstract
Proximal tubule (PT) cells are the most abundant cell type in the kidney cortex and critical for the re-absorption of water, ions, and small molecules like glucose and amino acids.These cells have a high demand for energy, and as a result have hundreds of mitochondria per cell. Mitochondrial copy number (mtCN) and DNA damage have been implicated as a source of dysfunction and decreased efficiency or even apoptosis in many cell types, including heart and brain cells. Here,we investigate the relationship between mitochondrial DNA (mtDNA)and nuclear DNA content as a method of assessing the role of mtCN in kidney function and aging.
mtDNA is present at up to hundreds of times greater levels compared to diploid nuclear DNA, which presents ac hallenge when trying to measure both targets in a single assay. Further, many current PCR-based assays do not allow for a level of sensitivity to make definitive conclusions about changes in mtCN.
Here, we aim to develop a fast and conclusive PCR-based assay to compare the abundance of mtDNA alongside nuclear DNA in rat kidney cortex. We selected two mtDNA deletion hotspots implicated in aging and disease, mt-Nd4and mt-Ndr. These hotspots are associated with the emergence of heteroplasmy and decreased mitochondrial function. Additionally, we selected a more stable mtDNA target, mt-Nd1 and a nuclear genomic target, Rpp30, to use as reference regions.
To overcome challenges with comparing these targets, we designed a 4-plex assay to target mt-Nd1, mt-Ndr, mt-Nd4 and Rpp30 with Countable PCR. We were able to achieve:
- Accurate,direct counts of mitochondrial copies per cell, with comparison between highabundance mtDNA targets and low abundance targetnuclear genome targets in the same reaction
- Lower variability in individual results, revealing subtle biological shifts not previously measured, including the initial increase in mtDNA counts associated with adolescence followed by a decline with age
- 4-target multiplex results without extensive optimization – three days to draw definitive conclusions about mtCN
Together, these results are an important proof-of-concept for tracing key mtDNA targets implicated in kidney disease or deteriorating function from aging. With the ability to compare mtDNA targets directly against their nuclear gene counterparts, we open up new avenues for interrogating the relationship between mtCN and mtDNA damage in a variety of cell types.

