Introduction
Viral genome integrity is a critical quality attribute (CQA) for ensuring the presence of functional viral elements that support effective outcomes in gene therapy. Researchers must monitor this CQA in order to make timely decisions about discarding low-quality batches and avoiding unnecessary time and resource expenditure.
Digital PCR (dPCR) is frequently used for assessing viral genome integrity. One approach is an indirect measure that employs a 2-plex assay that targets upstream (5’ end) and downstream (3’ end) of the transgene. The completeness of the viral genome is inferred by the ratio of the measured copies of the two regions.
The second dPCR-based approach uses DNA linkage analysis, which determines whether those 5’ and 3’ target regions are co-detected in the same molecule. However, to successfully apply DNA linkage analysis, the sample must be in a single-molecule regime. Conventional dPCR systems only have about 20,000 partitions, necessitating viral DNA samples to be diluted to <5000 copies per 20 µL [1]. This means samples must be diluted up to million-fold to avoid multi-occupancy of template molecules in each partition [2], resulting in a cumbersome and error-prone workflow. Without sufficient dilution, Poisson statistics do not apply, resulting in false positive complete genome assignment due to multi-occupancy of fragmented molecules, or under-quantification when multiple genome molecules occupy a single partition. Complex mathematical modelling is required to address the issue of multi-occupancy [3]. Overall, the limited dynamic range of dPCR systems restricts their practical use for linkage analysis for viral genome integrity measurements.
Countable PCR is an alternative platform for measuring viral genome integrity using DNA linkage, and represents a substantial improvement over alternatives. This technology enables single-molecule isolation of viral genome molecules in over 30 million compartments in 3D space, followed by lightsheet imaging that rapidly determines the co-occurrence of DNA targets on each molecule. This offers several improvements to dPCR that result in easier linkage analysis:
- The large number of compartments result in a broad dynamic range for single-molecule counting to reduce error and labor for serial dilutions.
- True single-molecule isolation enables precise determination of co-occupied (linked) viral genome targets.
- Countable Control Software delivers automatic linkage analysis, eliminating the need for manual thresholding and thereby reducing the time and subjectivity for data analysis
Here, we demonstrate the use of Countable Labs’ Universal Multiplex (UM) chemistry to design 3-plex integrity assays targeting various regions of the AAV genome, including the transgene. We then utilized the Countable System’s linkage module to measure AAV genome integrity via co-detection of the three regions on the vector genome.
Materials and methods
Materials
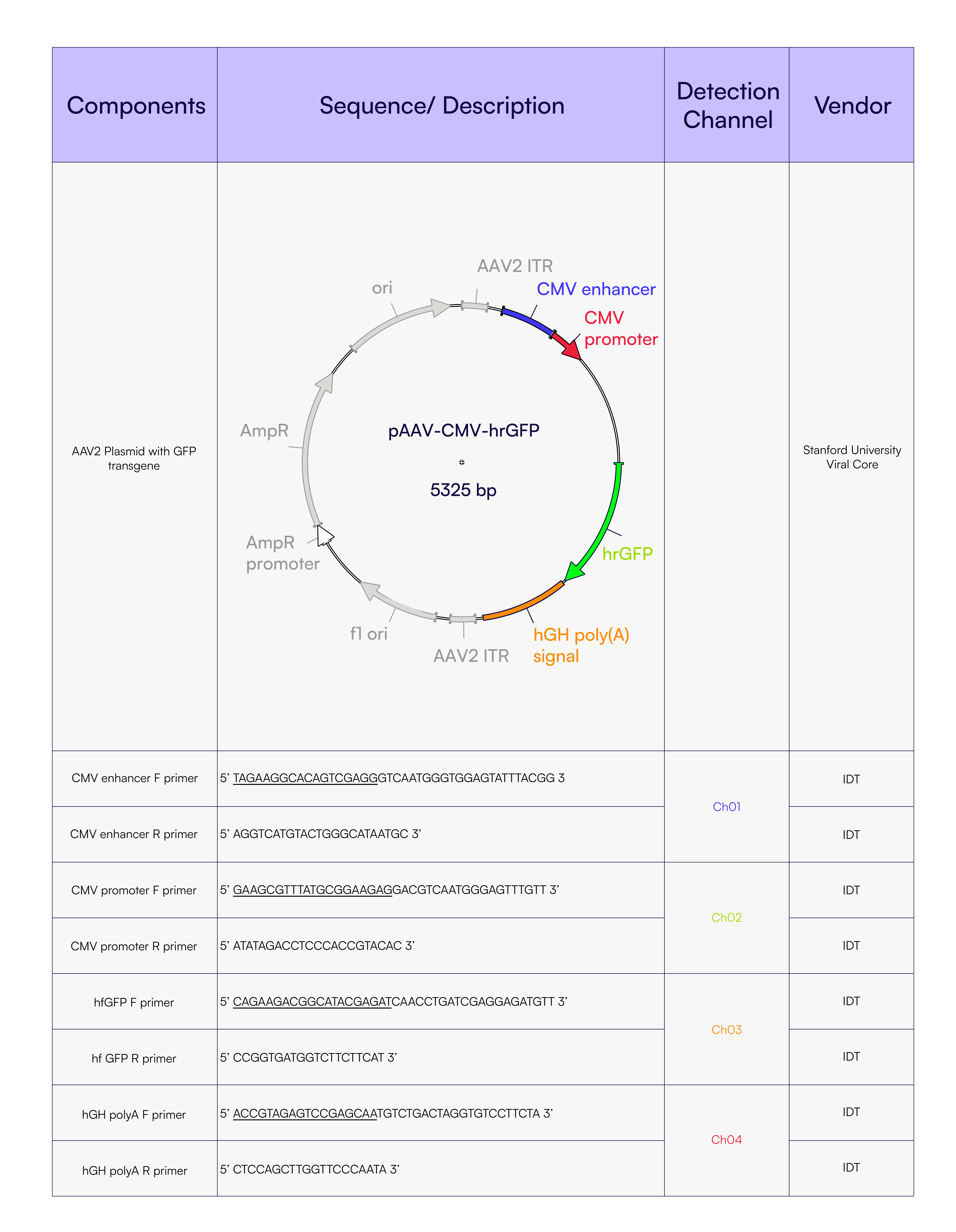
Each forward primer included a universal adapter for multiplex detection using the Universal Multiplex chemistry, enabling simultaneous measurement of multiple targets across the viral vector in a one-tube assay. The primer sequences are shown in Table 1.
Table 1. DNA Templates, Primers, and Probes. Underlined bases correspond to the UM adapter sequences.
Design of Countable PCR AAV genome integrity assay
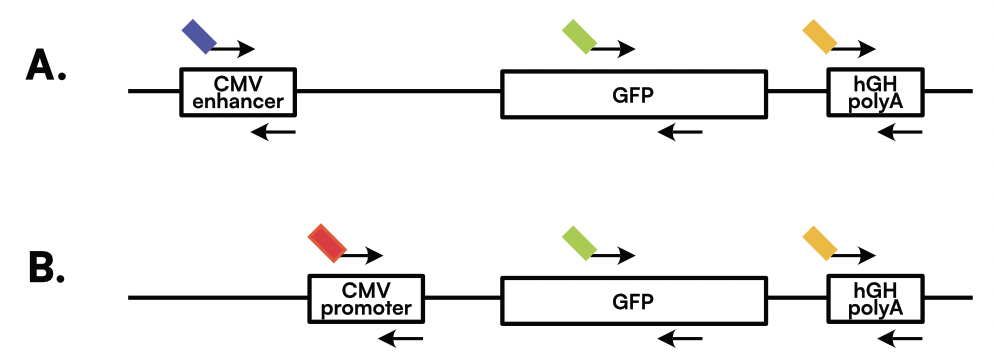
The experiments here were performed using two different configurations: 3-plex Integrity Assay A (Figure 1A) targeting the CMV enhancer on fluorescent detection channel 1 (Ch01), GFP transgene on Ch02, and the hGH polyA on Ch03; and 3-plex Integrity Assay B (Figure 1B) targeting the CMV promoter on Ch04, GFP transgene on Ch02, and the hGH polyA on Ch03. These targets were selected to span the viral vector: CMV enhancer/promoter near the 5’ end, GFP as the transgene in the middle, and hGH polyA near the 3’ end. This design allows for assessment of viral genome integrity by detecting the presence or absence of each target within each isolated single molecule. Full-length genomes will contain all 3 regions, while partial genomes will lack sequences at the 5’ or 3’ ends. These primers were designed by Countable Labs following best practices for multiplexing and avoiding primer-dimer interactions and hairpin structures.
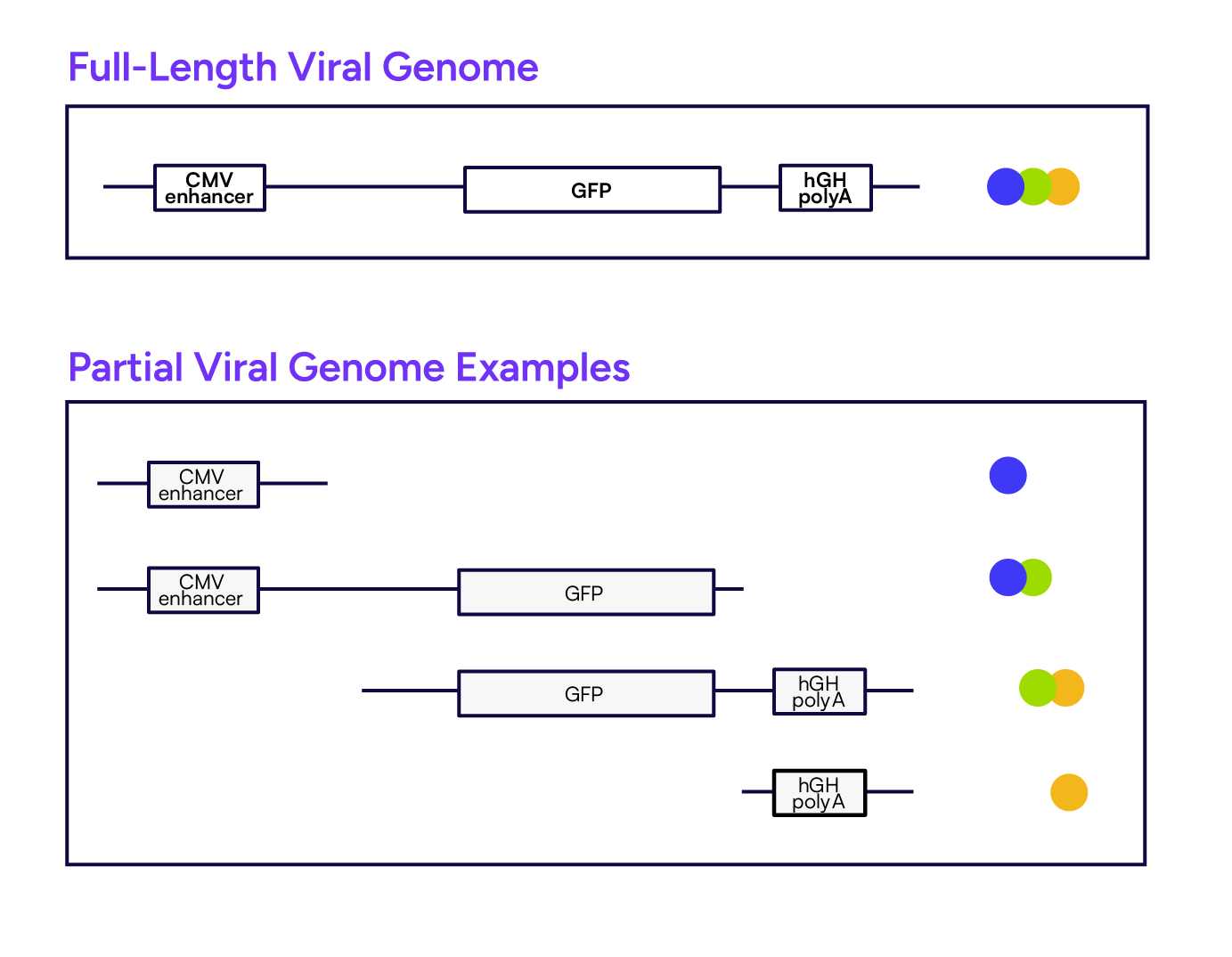
The linkage pattern detected in each partition depends on whether the viral genome is complete or partial. For example, a full-length viral genome will yield detection in all four targets across all four channels within the same partition (Figure 2). In contrast, partial genome fragments produce distinct signal subsets depending on which regions are retained.
Construction of synthetic proxies for complete and partial AAV genomes
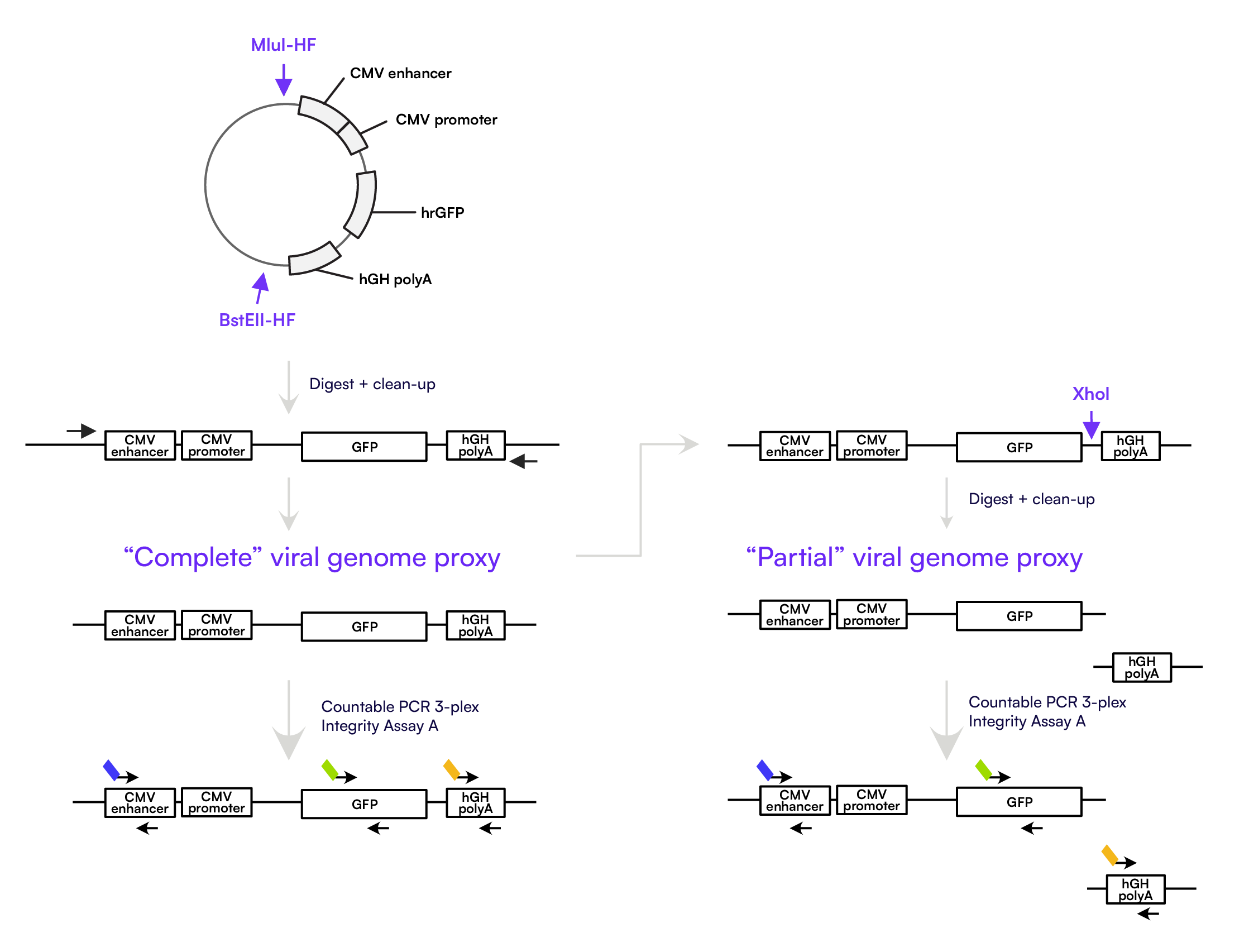
To evaluate the performance of the integrity assay, we used synthetic DNA proxies as shown in Figure 3.
To generate proxies for complete and partial AAV genomes for assay performance evaluation, we first digested the AAV2 plasmid with MluI-HF and BstEII-HF. This was followed by PCR amplification of the region spanning the CMV enhancer to the hGH polyA, producing a ~2kb amplicon representing a full-length genome.
To create partial genome proxies, the full-length amplicon was further digested with XhoI, yielding two fragments: one spanning the CMV enhancer to the GFP target, and another containing only the hGH polyA region. Defined mixtures of these “complete” and “partial” fragments were then prepared at varying ratios for performance testing on the Countable PCR platform using the Assay A configuration (CMV enhancer, GFP, hGH polyA).
Extraction of AAV genomic material
From a batch manufacturing event, after washing and pelleting culture cells that harbored the AAV vector, the pellet was resuspended in freezing buffer (20 mM Tris-HCl pH 8.0, 150 mM NaCl, 2 mM MgCl2 in water), freeze-thaw fractionated, and post-treated with Benzonase. This crude material was spun to remove debris, and the clarified lysate was retained. It was then further purified by iodixanol gradient ultracentrifugation to create the final AAV vector stock.
A small volume of this stock was extracted using a standard protocol [4]. In brief, the sample was treated with DNase I (50 units) at 37°C for 10 minutes, followed by heat inactivation at 72°C for 10 minutes. Proteinase K (0.4 units) was added to the sample and incubated at 50°C for 60 minutes, followed by heat inactivation at 95°C for 20 minutes. A final 10-fold dilution was applied to the extracted material before loading Countable PCR.
Countable PCR protocol
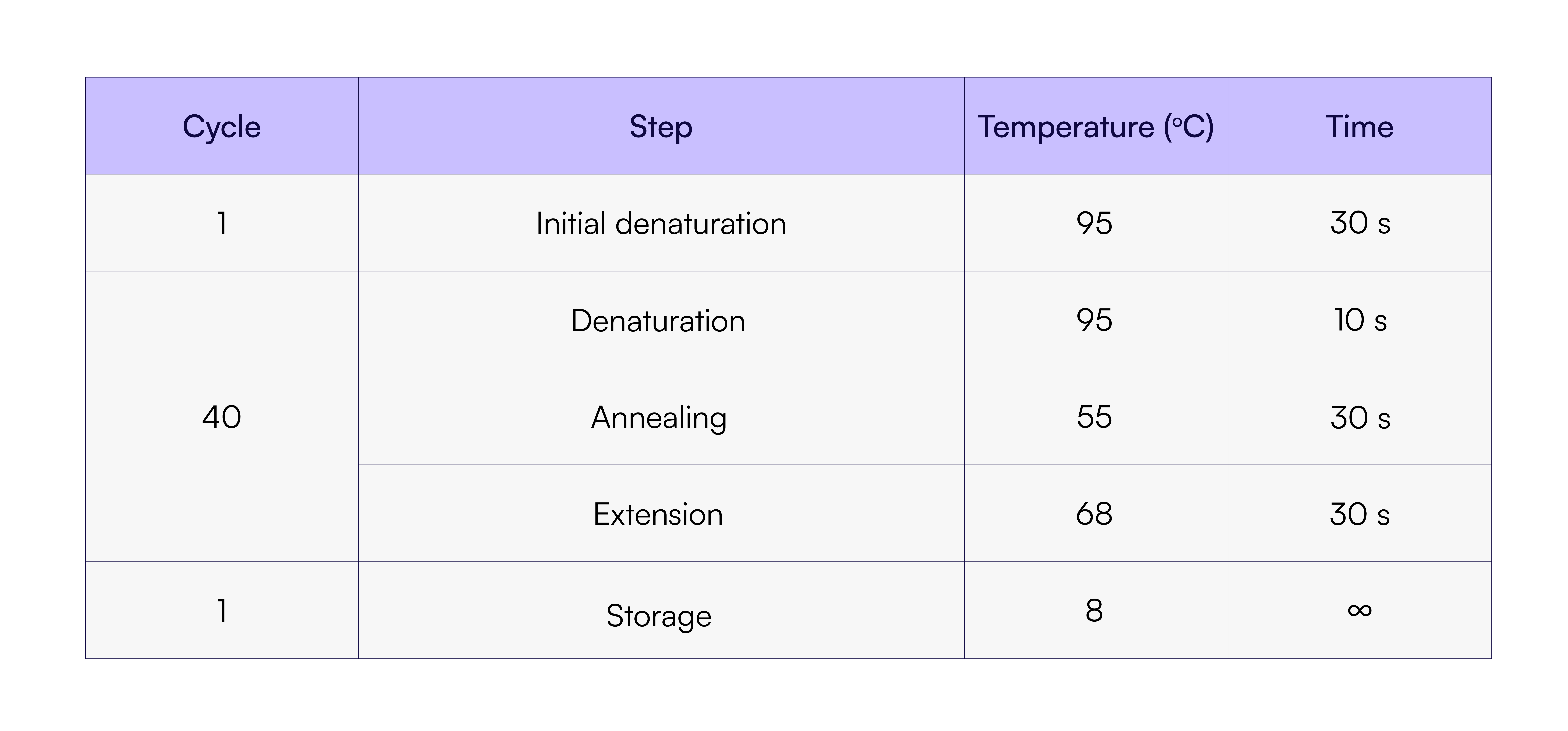
Following the Countable PCR Reaction Preparation User Guide, we set up a 3-plex Countable PCR reaction (targeting only the CMV enhancer, GFP, and hGH polyA regions), incorporating Universal Multiplexing Set A Kit for signal generation. The assay-specific thermal cycling protocol is shown in Table 2.
Table 2. Thermal cycling condition.
Measurement of target linkage on the Countable System
Countable PCR reactions were analyzed on the Countable System with the linkage module option enabled. The linked DNA target counts are found in the CountableLinkageSummary.csv output by the Countable analysis software.
The file summarizes the number of partitions that contain combinations of the target regions, which are used to identify either “complete” or “partial” viral genomes. Each row in the file corresponds to a sample, and each column corresponds to a specific target combination. With this 3-plex assay targeting the CMV enhancer (Ch01), GFP (Ch02), and hGH polyA (Ch03) regions:
- Counts for complete genomes (containing all three regions) are recorded under the column labeled Ch01_Ch02_Ch03
- Partial genomes spanning CMV to GFP are recorded under Ch01_Ch02
- Partial genomes containing only the polyA target are recorded under Ch03
To calculate the percentage of each genome type, the count in each relevant column is divided by the sum of all linkage pattern counts across that row.
Table 3. Example results from CountableLinkageSummary.csv

Using Sample1 as an example:
- “Complete” genome percentage: 63073/85901 x 100 = 73.43%
- “Partial” genome (CMV to GFP) percentage: 1756/85901 x 100 = 2.78%
- “Partial” genome (polyA only) percentage: 4401/85901 x 100 = 5.12%
Results
Countable PCR demonstrates robust single-plex performance for each viral genome target
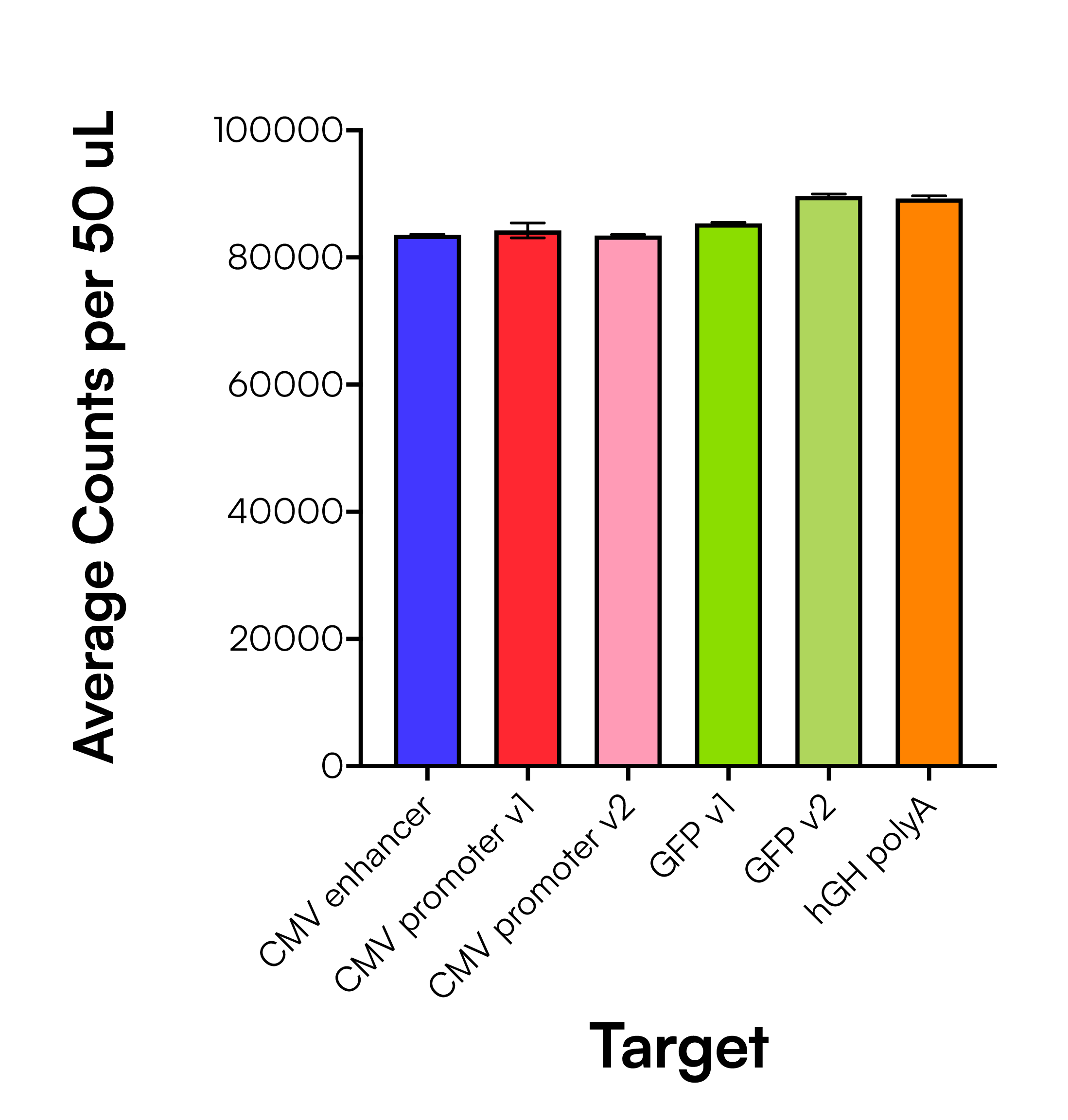
To ensure variability in integrity measurements was not due to target-dependent assay performance, we first evaluated the consistency of singleplex (1-plex) assays across multiple regions of the AAV genome. This test was performed using the “complete” AAV proxy material to verify that counts from each target region were comparable in the context of an intact genome. As shown in Figure 4, primer sets targeting the CMV enhancer, CMV promoter (2 versions), hrGFP (2 versions), and hGH polyA all produced highly consistent counts with minimal variation across replicates. These results confirm that the performance of Countable PCR’s assay using Universal Multiplexing chemistry is robust and uniform across different target regions, supporting the flexible design of multiplex integrity assays.
Countable PCR linkage analysis enables robust and precise quantification of linked genome fragments
We next applied the 3-plex integrity Assay A to defined mixtures of synthetic “complete” and “partial” AAV genome proxies that mimic varying levels of genome integrity. This multiplex assay targets the 5’ end (CMV enhancer), transgene (GFP), and 3’ end (hGH polyA) of the viral genome, enabling reliable discrimination between complete and partial genomes. Additionally, minimal optimization was required to adapt this assay to a multiplexed format. Countable PCR’s matrix captures targets in a true single-molecule fashion, preventing co-occupancy of fragmented targets and ensuring accurate quantification of genome integrity.
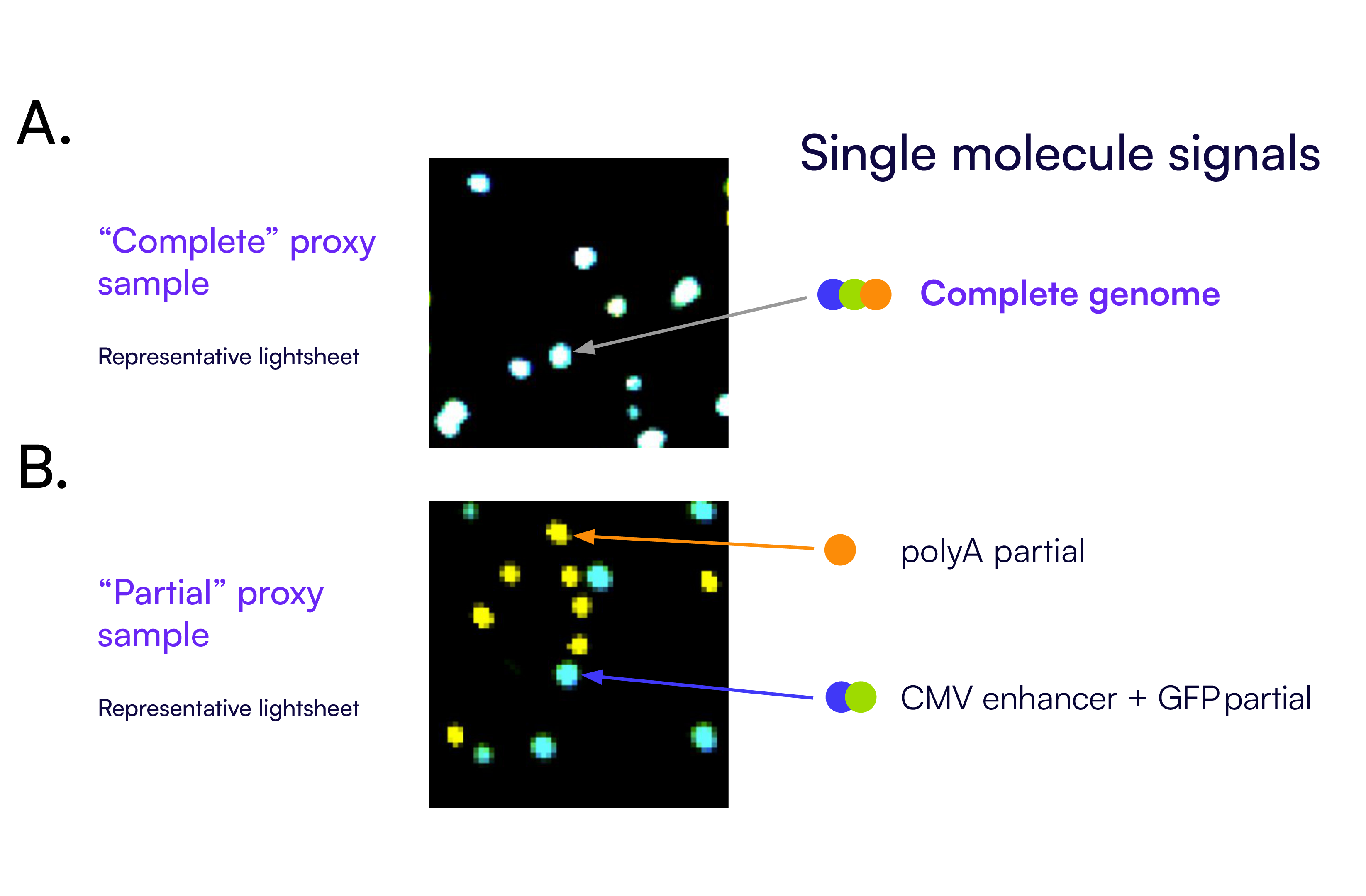
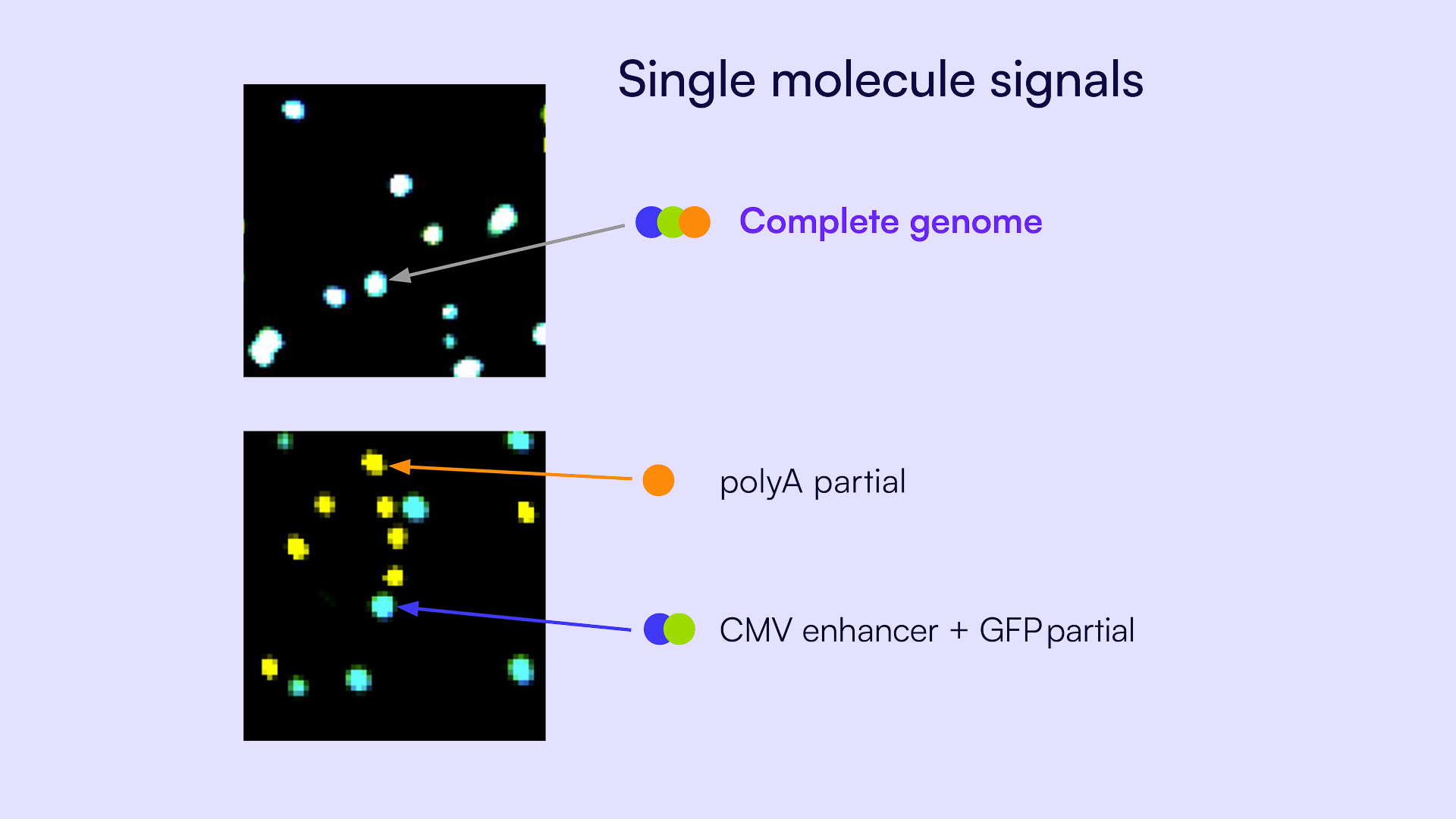
As shown in Figure 5, individual partitions contain distinct signal combinations based on which regions of the genome are present. Complete viral genome fragments generate co-detection across all three targets (Figure 5A) while partial genomes display detection patterns corresponding to only one or two targets (Figure 5B). These signal combinations corresponding to different linkage patterns are the basis for the linkage summary analysis. The signal patterns are counted and categorized to determine the total number of complete and partial genome fragments in each reaction.
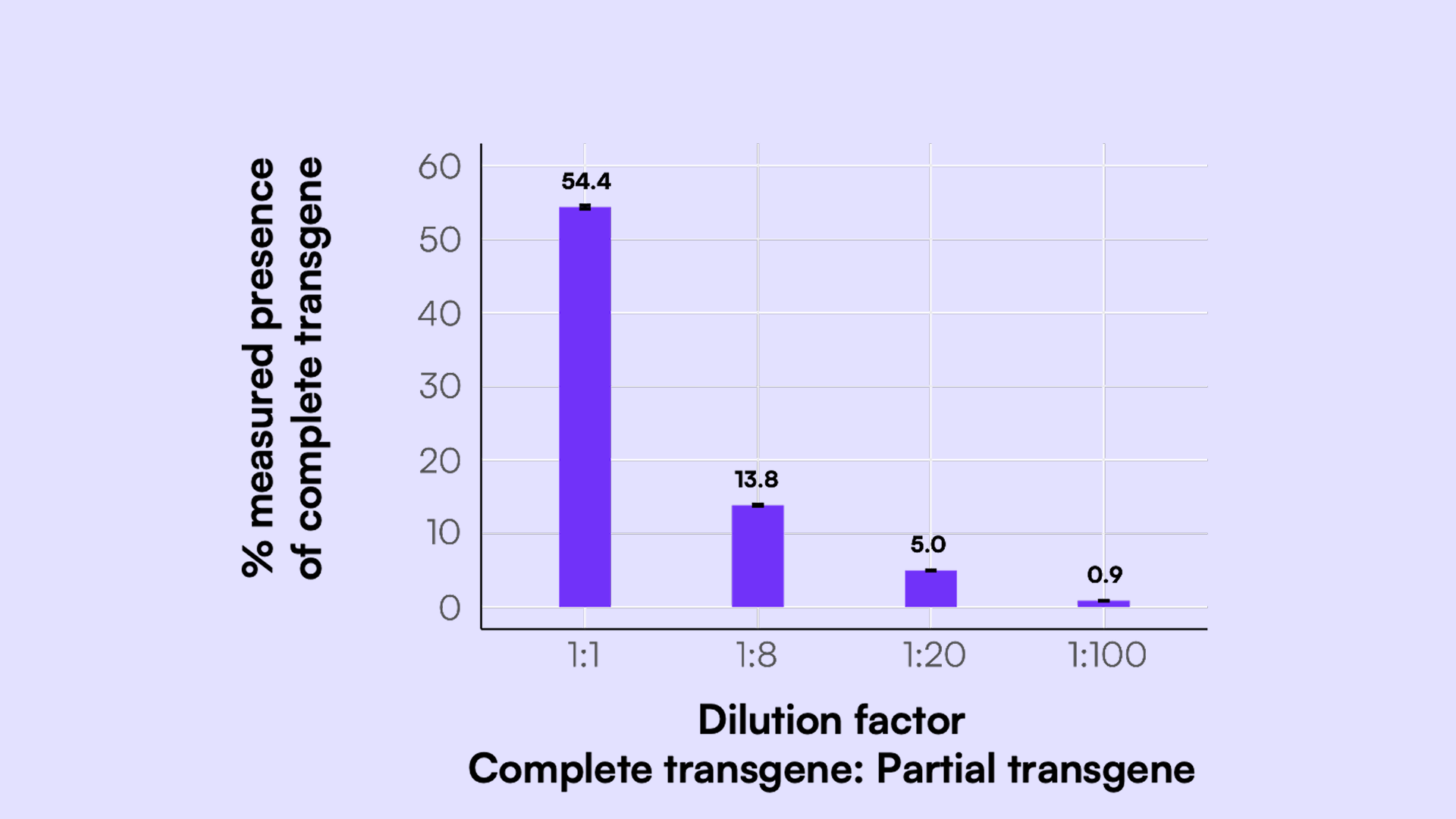
Using these categorized counts, we calculated the percentage of each genome type per sample, as shown in Figure 6. When tested across a range of defined complete:partial input ratios, the assay captured expected trends: the proportion of complete genome decreased with lower input levels of the complete template while partial genome signals correspondingly increased.
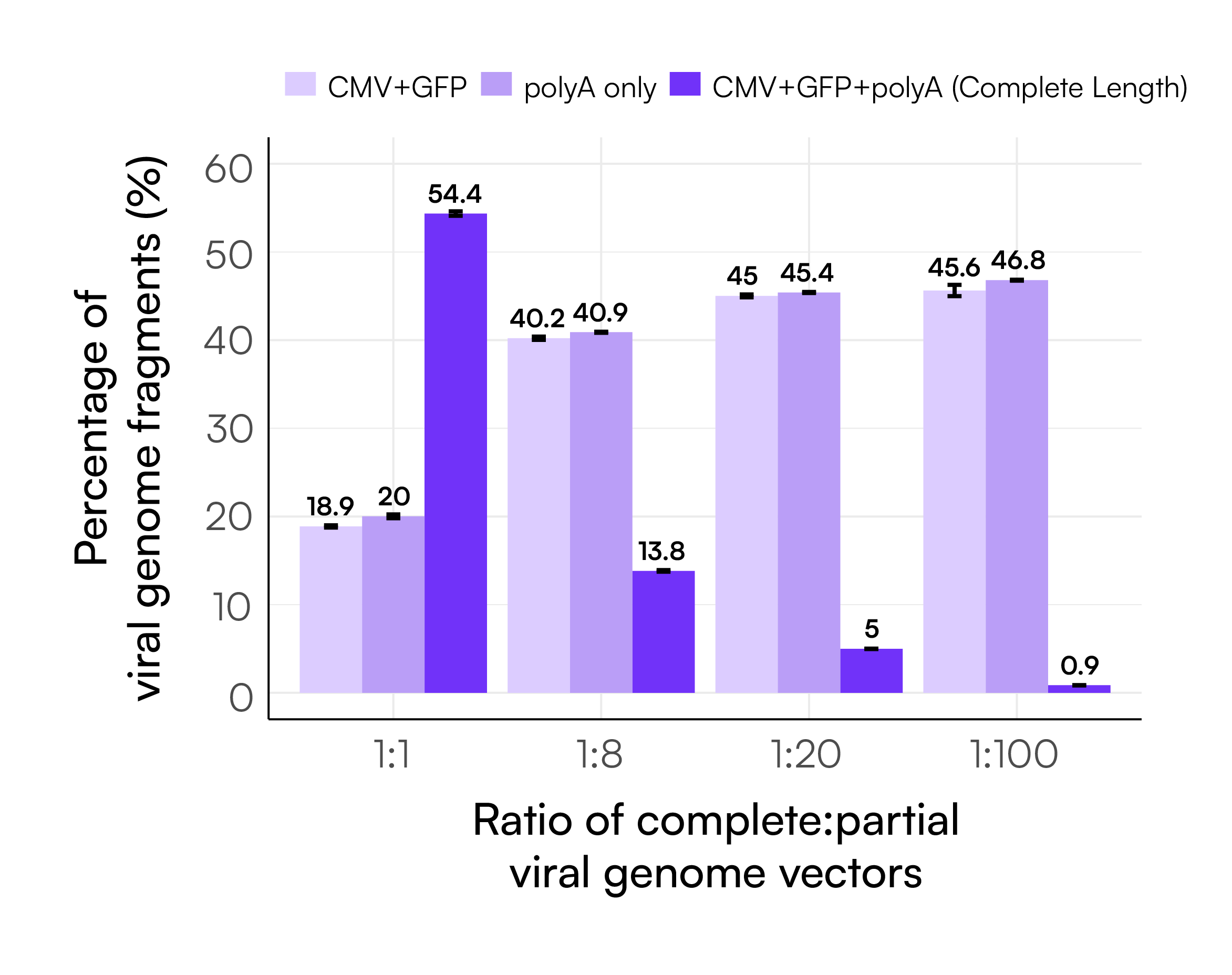
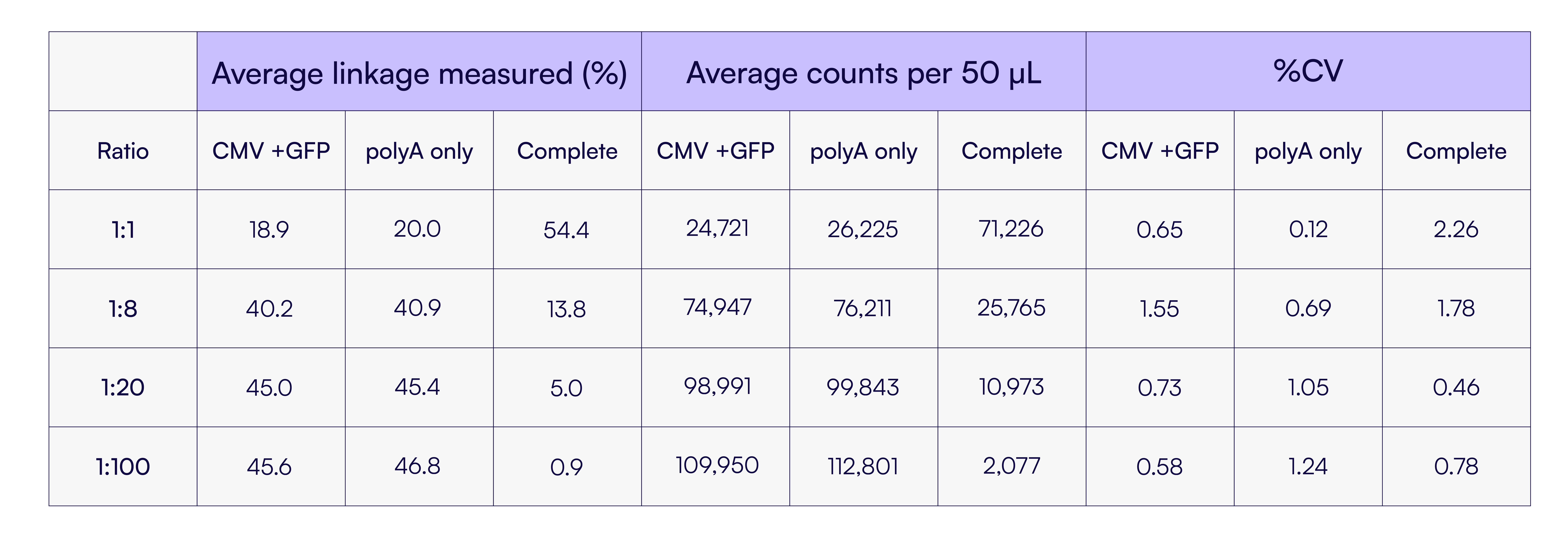
Notably, the percentages of CMV+GFP and polyA-only signals remained nearly identical across all sample ratios. This is expected based on the restriction enzyme digest used to generate the partial genome proxies, which yields one fragment spanning CMV to GFP and another containing only the polyA region. The balanced representation of these two fragment types confirms the assay’s quantitative accuracy. Table 4 further highlights the high precision of these measurements, with all %CVs under 3%.
Table 4. Summary of the average linkage percentages, counts, and CVs from the different ratios of complete vs partial genome experiments.
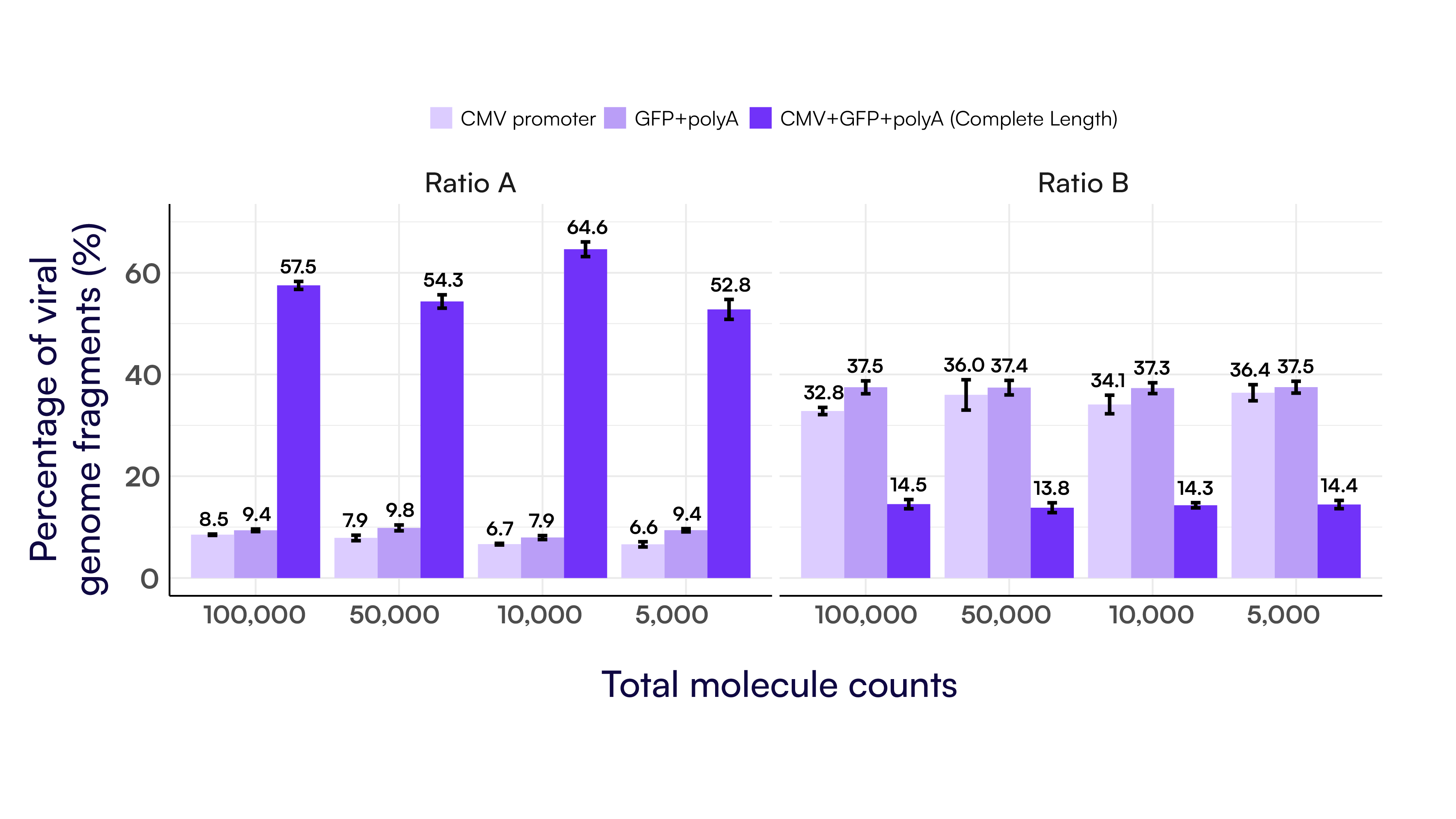
To further evaluate the performance of this assay, we tested two genome integrity ratios across four input levels (100,000 to 5,000 molecules) using the 3-plex Integrity Assay B. This assay targets the 5’ end (CMV promoter), transgene (GFP), and 3’ end (hGH polyA) of the viral genome. As shown in Figure 7, the observed percentages of complete and partial genomes remained consistent across the dilution series. This demonstrates that Countable PCR provides reliable, quantitative genome integrity measurements across a broad dynamic range.
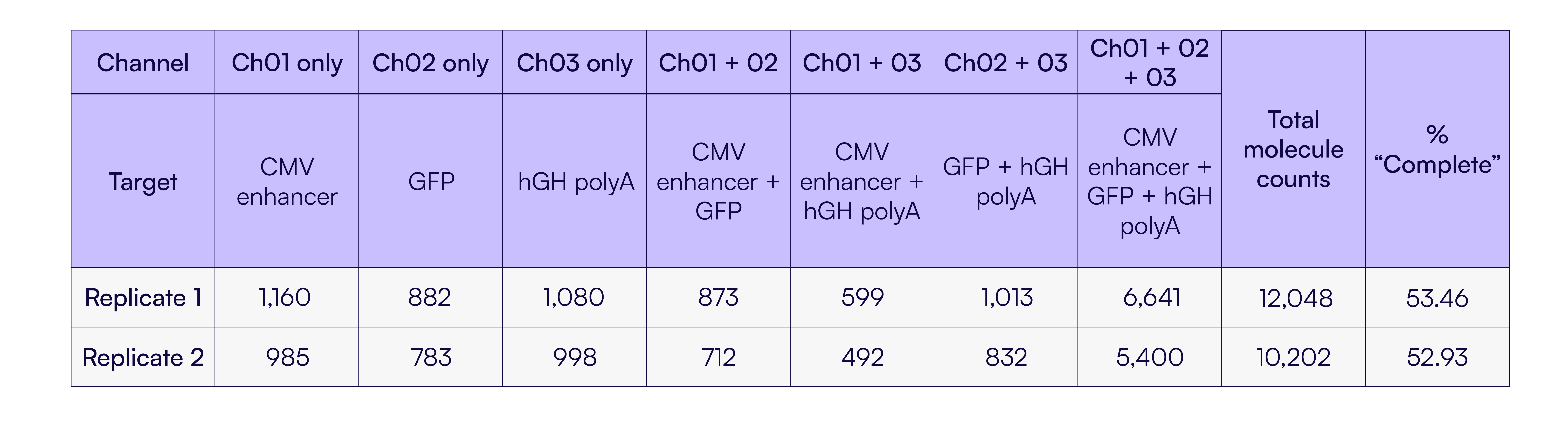
Having established the performance of the assay using synthetic proxies, we next applied it to a purified AAV sample to assess genome integrity in a native context. Testing this extracted sample on the Countable Labs 3-plex Integrity Assay A showed ~53% of the transgene molecules were fully intact (Table 5), from the CMV enhancer to the poly A region. This was consistent with orthogonal methods of integrity verification, such as analytical ultracentrifugation (AUC) and anion exchange chromatography (AEX), which typically place the full-length fraction of AAV preps at ~50-70% [5,6]. This concordance highlights the utility of the Countable assay as a quantitative method for assessing AAV genome integrity.
Table 5. Detection of full-length AAV genomes from purified AAV samples using Countable PCR. Linkage summary output for a purified AAV sample analyzed with Countable Lab’s 3-plex Integrity assay. Two replicates are shown, with the proportion of complete genomes being ~53% in both replicates. These results are consistent with the expected ranges for full-length genome content based on orthogonal AAV characterization methods.
Conclusion
Countable PCR offers a novel approach for cell and gene therapy researchers seeking to accurately and quantifiably assess the genomic integrity of viral vectors by directly measuring the linkage of genomic regions on a viral DNA molecule. Countable PCR’s platform achieves this by:
- Offering a broad dynamic range to avoid error and bias from excessive dilution
- Enabling true single-molecule isolation in compartments resulting in low-interference linkage assignment
- Providing automatic counts of each target molecule, including their linkage assignments, avoiding the subjectivity of manual thresholding
Further, the low cost and versatility of Universal Multiplexing (UM) chemistry make it easy to design assays specific to transgene targets, enabling rapid turnaround time in developing genome integrity assays that incorporate the transgene for improved measurement reliability.
Countable PCR offers a robust and flexible pathway to viral genome linkage analysis. With direct counts and fast turnaround times, Countable PCR represents a more accurate and powerful tool for finding critical quality attributes.
References
- <span id="resource-1"></span>Bio-Rad Laboratories. Droplet Digital™ PCR Applications Guide. Bulletin 6407. Accessed July 11, 2025. Available from: https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6407.pdf
- <span id="resource-2"></span>Addgene. Droplet Digital PCR for AAV Quantitation. Addgene Blog. 2020 Jan 16. Available from: https://blog.addgene.org/droplet-digital-pcr-for-aav-quantitation
- <span id="resource-3"></span>Duong T, Firmo M, Li CT, Gu B, Wang P. Three-dimensional linkage analysis with digital PCR for genome integrity and identity of recombinant adeno-associated virus. Sci Rep. 2025;15(1):2154. doi:10.1038/s41598-024-77378-7.
- <span id="resource-4"></span>Werling NJ, Satkunanathan S, Thorpe R, Zhao Y. Systematic Comparison and Validation of Quantitative Real-Time PCR Methods for the Quantitation of Adeno-Associated Viral Products. Hum Gene Ther Methods. 2015 Jun;26(3):82-92. doi: 10.1089/hgtb.2015.013. Epub 2015 Jun 9. PMID: 25953194; PMCID: PMC4492554.
- <span id="resource-5"></span>McColl-Carboni A, Dollive S, Laughlin S, Lushi R, MacArthur M, Zhou S, Gagnon J, Smith CA, Burnham B, Horton R, Lata D, Uga B, Natu K, Michel E, Slater C, DaSilva E, Bruccoleri R, Kelly T, McGivney JB 4th. Analytical characterization of full, intermediate, and empty AAV capsids. Gene Ther. 2024 May;31(5-6):285-294. doi: 10.1038/s41434-024-00444-2. Epub 2024 Feb 19. PMID: 38374348; PMCID: PMC11090809.
- <span id="resource-6"></span>Wang C, Mulagapati SHR, Chen Z, Du J, Zhao X, Xi G, Chen L, Linke T, Gao C, Schmelzer AE, Liu D. Developing an Anion Exchange Chromatography Assay for Determining Empty and Full Capsid Contents in AAV6.2. Mol Ther Methods Clin Dev. 2019 Sep 26;15:257-263. doi: 10.1016/j.omtm.2019.09.006. PMID: 31720304; PMCID: PMC6838793.
Introduction
Viral genome integrity is a critical quality attribute (CQA) for ensuring the presence of functional viral elements that support effective outcomes in gene therapy. Researchers must monitor this CQA in order to make timely decisions about discarding low-quality batches and avoiding unnecessary time and resource expenditure.
Digital PCR (dPCR) is frequently used for assessing viral genome integrity. One approach is an indirect measure that employs a 2-plex assay that targets upstream (5’ end) and downstream (3’ end) of the transgene. The completeness of the viral genome is inferred by the ratio of the measured copies of the two regions.
The second dPCR-based approach uses DNA linkage analysis, which determines whether those 5’ and 3’ target regions are co-detected in the same molecule. However, to successfully apply DNA linkage analysis, the sample must be in a single-molecule regime. Conventional dPCR systems only have about 20,000 partitions, necessitating viral DNA samples to be diluted to <5000 copies per 20 µL [1]. This means samples must be diluted up to million-fold to avoid multi-occupancy of template molecules in each partition [2], resulting in a cumbersome and error-prone workflow. Without sufficient dilution, Poisson statistics do not apply, resulting in false positive complete genome assignment due to multi-occupancy of fragmented molecules, or under-quantification when multiple genome molecules occupy a single partition. Complex mathematical modelling is required to address the issue of multi-occupancy [3]. Overall, the limited dynamic range of dPCR systems restricts their practical use for linkage analysis for viral genome integrity measurements.
Countable PCR is an alternative platform for measuring viral genome integrity using DNA linkage, and represents a substantial improvement over alternatives. This technology enables single-molecule isolation of viral genome molecules in over 30 million compartments in 3D space, followed by lightsheet imaging that rapidly determines the co-occurrence of DNA targets on each molecule. This offers several improvements to dPCR that result in easier linkage analysis:
- The large number of compartments result in a broad dynamic range for single-molecule counting to reduce error and labor for serial dilutions.
- True single-molecule isolation enables precise determination of co-occupied (linked) viral genome targets.
- Countable Control Software delivers automatic linkage analysis, eliminating the need for manual thresholding and thereby reducing the time and subjectivity for data analysis
Here, we demonstrate the use of Countable Labs’ Universal Multiplex (UM) chemistry to design 3-plex integrity assays targeting various regions of the AAV genome, including the transgene. We then utilized the Countable System’s linkage module to measure AAV genome integrity via co-detection of the three regions on the vector genome.
Materials and methods
Materials
Each forward primer included a universal adapter for multiplex detection using the Universal Multiplex chemistry, enabling simultaneous measurement of multiple targets across the viral vector in a one-tube assay. The primer sequences are shown in Table 1.
Table 1. DNA Templates, Primers, and Probes. Underlined bases correspond to the UM adapter sequences.

Design of Countable PCR AAV genome integrity assay
The experiments here were performed using two different configurations: 3-plex Integrity Assay A (Figure 1A) targeting the CMV enhancer on fluorescent detection channel 1 (Ch01), GFP transgene on Ch02, and the hGH polyA on Ch03; and 3-plex Integrity Assay B (Figure 1B) targeting the CMV promoter on Ch04, GFP transgene on Ch02, and the hGH polyA on Ch03. These targets were selected to span the viral vector: CMV enhancer/promoter near the 5’ end, GFP as the transgene in the middle, and hGH polyA near the 3’ end. This design allows for assessment of viral genome integrity by detecting the presence or absence of each target within each isolated single molecule. Full-length genomes will contain all 3 regions, while partial genomes will lack sequences at the 5’ or 3’ ends. These primers were designed by Countable Labs following best practices for multiplexing and avoiding primer-dimer interactions and hairpin structures.

The linkage pattern detected in each partition depends on whether the viral genome is complete or partial. For example, a full-length viral genome will yield detection in all four targets across all four channels within the same partition (Figure 2). In contrast, partial genome fragments produce distinct signal subsets depending on which regions are retained.

Construction of synthetic proxies for complete and partial AAV genomes
To evaluate the performance of the integrity assay, we used synthetic DNA proxies as shown in Figure 3.
To generate proxies for complete and partial AAV genomes for assay performance evaluation, we first digested the AAV2 plasmid with MluI-HF and BstEII-HF. This was followed by PCR amplification of the region spanning the CMV enhancer to the hGH polyA, producing a ~2kb amplicon representing a full-length genome.
To create partial genome proxies, the full-length amplicon was further digested with XhoI, yielding two fragments: one spanning the CMV enhancer to the GFP target, and another containing only the hGH polyA region. Defined mixtures of these “complete” and “partial” fragments were then prepared at varying ratios for performance testing on the Countable PCR platform using the Assay A configuration (CMV enhancer, GFP, hGH polyA).

Extraction of AAV genomic material
From a batch manufacturing event, after washing and pelleting culture cells that harbored the AAV vector, the pellet was resuspended in freezing buffer (20 mM Tris-HCl pH 8.0, 150 mM NaCl, 2 mM MgCl2 in water), freeze-thaw fractionated, and post-treated with Benzonase. This crude material was spun to remove debris, and the clarified lysate was retained. It was then further purified by iodixanol gradient ultracentrifugation to create the final AAV vector stock.
A small volume of this stock was extracted using a standard protocol [4]. In brief, the sample was treated with DNase I (50 units) at 37°C for 10 minutes, followed by heat inactivation at 72°C for 10 minutes. Proteinase K (0.4 units) was added to the sample and incubated at 50°C for 60 minutes, followed by heat inactivation at 95°C for 20 minutes. A final 10-fold dilution was applied to the extracted material before loading Countable PCR.
Countable PCR protocol
Following the Countable PCR Reaction Preparation User Guide, we set up a 3-plex Countable PCR reaction (targeting only the CMV enhancer, GFP, and hGH polyA regions), incorporating Universal Multiplexing Set A Kit for signal generation. The assay-specific thermal cycling protocol is shown in Table 2.
Table 2. Thermal cycling condition.

Measurement of target linkage on the Countable System
Countable PCR reactions were analyzed on the Countable System with the linkage module option enabled. The linked DNA target counts are found in the CountableLinkageSummary.csv output by the Countable analysis software.
The file summarizes the number of partitions that contain combinations of the target regions, which are used to identify either “complete” or “partial” viral genomes. Each row in the file corresponds to a sample, and each column corresponds to a specific target combination. With this 3-plex assay targeting the CMV enhancer (Ch01), GFP (Ch02), and hGH polyA (Ch03) regions:
- Counts for complete genomes (containing all three regions) are recorded under the column labeled Ch01_Ch02_Ch03
- Partial genomes spanning CMV to GFP are recorded under Ch01_Ch02
- Partial genomes containing only the polyA target are recorded under Ch03
To calculate the percentage of each genome type, the count in each relevant column is divided by the sum of all linkage pattern counts across that row.
Table 3. Example results from CountableLinkageSummary.csv

Using Sample1 as an example:
- “Complete” genome percentage: 63073/85901 x 100 = 73.43%
- “Partial” genome (CMV to GFP) percentage: 1756/85901 x 100 = 2.78%
- “Partial” genome (polyA only) percentage: 4401/85901 x 100 = 5.12%
Results
Countable PCR demonstrates robust single-plex performance for each viral genome target
To ensure variability in integrity measurements was not due to target-dependent assay performance, we first evaluated the consistency of singleplex (1-plex) assays across multiple regions of the AAV genome. This test was performed using the “complete” AAV proxy material to verify that counts from each target region were comparable in the context of an intact genome. As shown in Figure 4, primer sets targeting the CMV enhancer, CMV promoter (2 versions), hrGFP (2 versions), and hGH polyA all produced highly consistent counts with minimal variation across replicates. These results confirm that the performance of Countable PCR’s assay using Universal Multiplexing chemistry is robust and uniform across different target regions, supporting the flexible design of multiplex integrity assays.

Countable PCR linkage analysis enables robust and precise quantification of linked genome fragments
We next applied the 3-plex integrity Assay A to defined mixtures of synthetic “complete” and “partial” AAV genome proxies that mimic varying levels of genome integrity. This multiplex assay targets the 5’ end (CMV enhancer), transgene (GFP), and 3’ end (hGH polyA) of the viral genome, enabling reliable discrimination between complete and partial genomes. Additionally, minimal optimization was required to adapt this assay to a multiplexed format. Countable PCR’s matrix captures targets in a true single-molecule fashion, preventing co-occupancy of fragmented targets and ensuring accurate quantification of genome integrity.
As shown in Figure 5, individual partitions contain distinct signal combinations based on which regions of the genome are present. Complete viral genome fragments generate co-detection across all three targets (Figure 5A) while partial genomes display detection patterns corresponding to only one or two targets (Figure 5B). These signal combinations corresponding to different linkage patterns are the basis for the linkage summary analysis. The signal patterns are counted and categorized to determine the total number of complete and partial genome fragments in each reaction.

Using these categorized counts, we calculated the percentage of each genome type per sample, as shown in Figure 6. When tested across a range of defined complete:partial input ratios, the assay captured expected trends: the proportion of complete genome decreased with lower input levels of the complete template while partial genome signals correspondingly increased.

Notably, the percentages of CMV+GFP and polyA-only signals remained nearly identical across all sample ratios. This is expected based on the restriction enzyme digest used to generate the partial genome proxies, which yields one fragment spanning CMV to GFP and another containing only the polyA region. The balanced representation of these two fragment types confirms the assay’s quantitative accuracy. Table 4 further highlights the high precision of these measurements, with all %CVs under 3%.
Table 4. Summary of the average linkage percentages, counts, and CVs from the different ratios of complete vs partial genome experiments.

To further evaluate the performance of this assay, we tested two genome integrity ratios across four input levels (100,000 to 5,000 molecules) using the 3-plex Integrity Assay B. This assay targets the 5’ end (CMV promoter), transgene (GFP), and 3’ end (hGH polyA) of the viral genome. As shown in Figure 7, the observed percentages of complete and partial genomes remained consistent across the dilution series. This demonstrates that Countable PCR provides reliable, quantitative genome integrity measurements across a broad dynamic range.

Having established the performance of the assay using synthetic proxies, we next applied it to a purified AAV sample to assess genome integrity in a native context. Testing this extracted sample on the Countable Labs 3-plex Integrity Assay A showed ~53% of the transgene molecules were fully intact (Table 5), from the CMV enhancer to the poly A region. This was consistent with orthogonal methods of integrity verification, such as analytical ultracentrifugation (AUC) and anion exchange chromatography (AEX), which typically place the full-length fraction of AAV preps at ~50-70% [5,6]. This concordance highlights the utility of the Countable assay as a quantitative method for assessing AAV genome integrity.
Table 5. Detection of full-length AAV genomes from purified AAV samples using Countable PCR. Linkage summary output for a purified AAV sample analyzed with Countable Lab’s 3-plex Integrity assay. Two replicates are shown, with the proportion of complete genomes being ~53% in both replicates. These results are consistent with the expected ranges for full-length genome content based on orthogonal AAV characterization methods.

Conclusion
Countable PCR offers a novel approach for cell and gene therapy researchers seeking to accurately and quantifiably assess the genomic integrity of viral vectors by directly measuring the linkage of genomic regions on a viral DNA molecule. Countable PCR’s platform achieves this by:
- Offering a broad dynamic range to avoid error and bias from excessive dilution
- Enabling true single-molecule isolation in compartments resulting in low-interference linkage assignment
- Providing automatic counts of each target molecule, including their linkage assignments, avoiding the subjectivity of manual thresholding
Further, the low cost and versatility of Universal Multiplexing (UM) chemistry make it easy to design assays specific to transgene targets, enabling rapid turnaround time in developing genome integrity assays that incorporate the transgene for improved measurement reliability.
Countable PCR offers a robust and flexible pathway to viral genome linkage analysis. With direct counts and fast turnaround times, Countable PCR represents a more accurate and powerful tool for finding critical quality attributes.
References
- <span id="resource-1"></span>Bio-Rad Laboratories. Droplet Digital™ PCR Applications Guide. Bulletin 6407. Accessed July 11, 2025. Available from: https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6407.pdf
- <span id="resource-2"></span>Addgene. Droplet Digital PCR for AAV Quantitation. Addgene Blog. 2020 Jan 16. Available from: https://blog.addgene.org/droplet-digital-pcr-for-aav-quantitation
- <span id="resource-3"></span>Duong T, Firmo M, Li CT, Gu B, Wang P. Three-dimensional linkage analysis with digital PCR for genome integrity and identity of recombinant adeno-associated virus. Sci Rep. 2025;15(1):2154. doi:10.1038/s41598-024-77378-7.
- <span id="resource-4"></span>Werling NJ, Satkunanathan S, Thorpe R, Zhao Y. Systematic Comparison and Validation of Quantitative Real-Time PCR Methods for the Quantitation of Adeno-Associated Viral Products. Hum Gene Ther Methods. 2015 Jun;26(3):82-92. doi: 10.1089/hgtb.2015.013. Epub 2015 Jun 9. PMID: 25953194; PMCID: PMC4492554.
- <span id="resource-5"></span>McColl-Carboni A, Dollive S, Laughlin S, Lushi R, MacArthur M, Zhou S, Gagnon J, Smith CA, Burnham B, Horton R, Lata D, Uga B, Natu K, Michel E, Slater C, DaSilva E, Bruccoleri R, Kelly T, McGivney JB 4th. Analytical characterization of full, intermediate, and empty AAV capsids. Gene Ther. 2024 May;31(5-6):285-294. doi: 10.1038/s41434-024-00444-2. Epub 2024 Feb 19. PMID: 38374348; PMCID: PMC11090809.
- <span id="resource-6"></span>Wang C, Mulagapati SHR, Chen Z, Du J, Zhao X, Xi G, Chen L, Linke T, Gao C, Schmelzer AE, Liu D. Developing an Anion Exchange Chromatography Assay for Determining Empty and Full Capsid Contents in AAV6.2. Mol Ther Methods Clin Dev. 2019 Sep 26;15:257-263. doi: 10.1016/j.omtm.2019.09.006. PMID: 31720304; PMCID: PMC6838793.



.png)